Development of the Cochrane Collaboration's Central Register of Controlled Clinical Trials | Semantic Scholar

Impact of blinding on estimated treatment effects in randomised clinical trials: meta-epidemiological study | The BMJ

Cochrane supports European regulators as they urge clinical trial sponsors to share their results | Cochrane
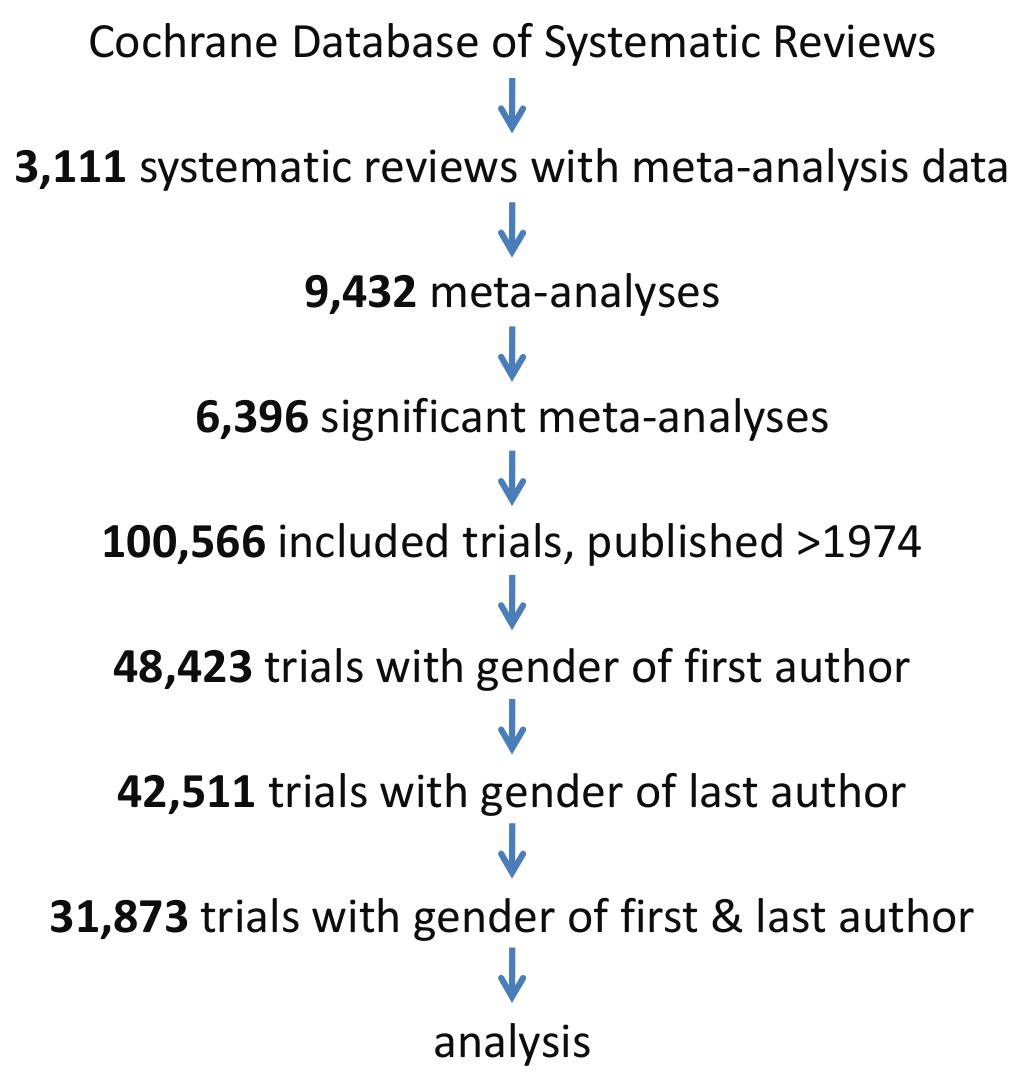
Research: Adequate statistical power in clinical trials is associated with the combination of a male first author and a female last author | eLife

THE COCHRANE LIBRARY ON WILEY INTERSCIENCE. Presentation Agenda Brief introduction of Evidence-Based Medicine theories The Cochrane Collaboration – origins, - ppt download

The Cochrane Library on Twitter: "New monitoring strategies for # clinicaltrials - https://t.co/fMuuJIZcDs New @cochranemthds review includes 8 studies, covering various monitoring strategies in a wide range of clinical trials, including national &

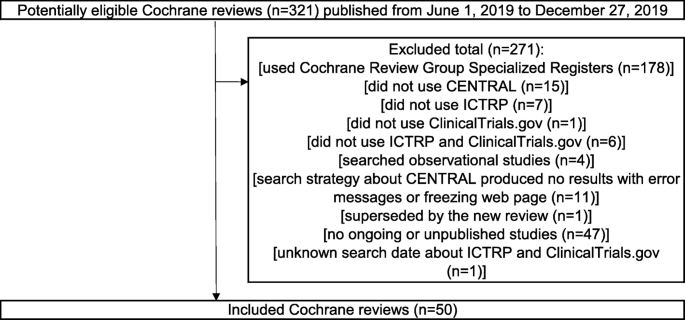
Flow chart of study selection process. Abbreviations: CDSR, Cochrane... | Download Scientific Diagram
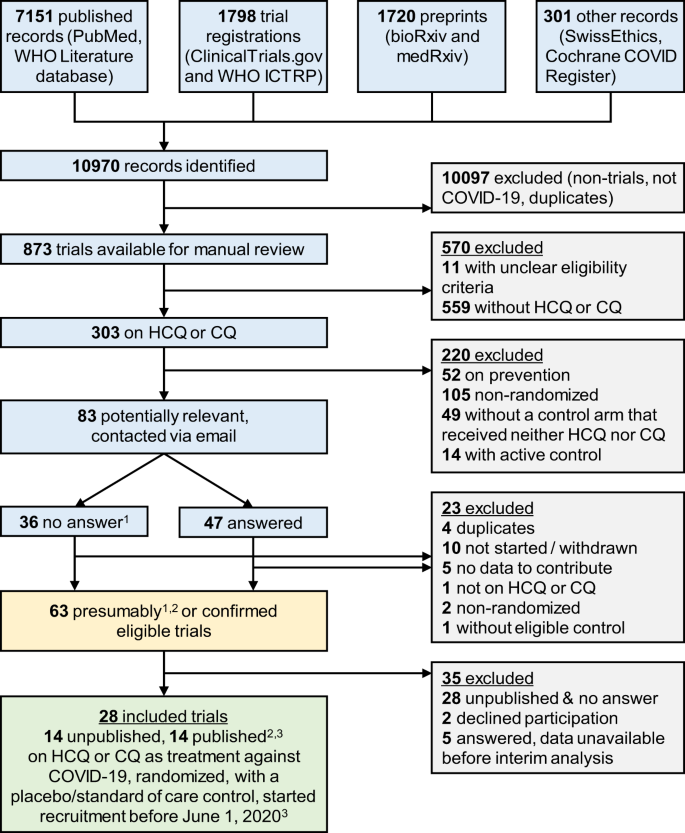
Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19 from an international collaborative meta-analysis of randomized trials | Nature Communications