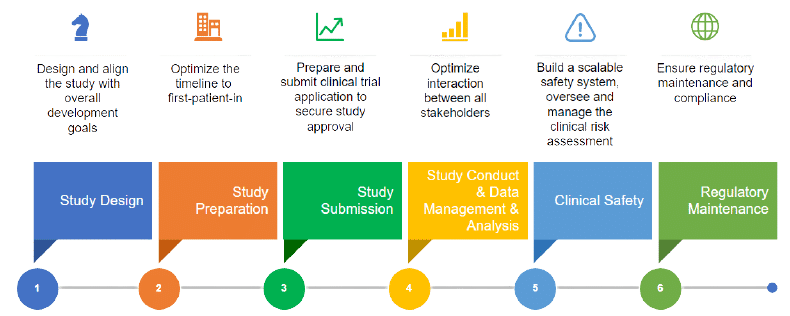
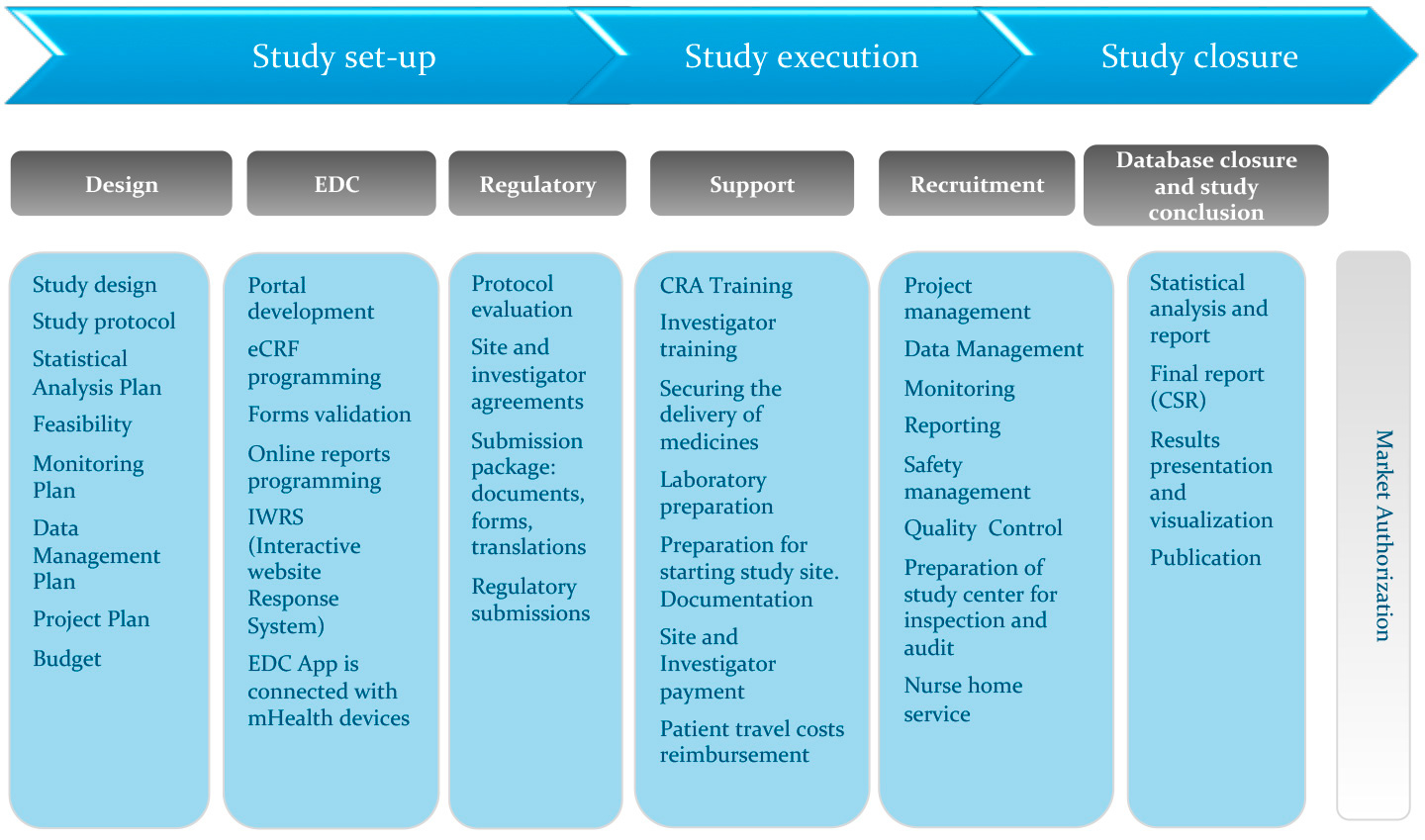
Drug Safety Archives - Qtech-Sol USA offers Clinical Research / Trials, Pharmacovigilance, Drug Safety, Clinical Data Management, Clinical SAS Programming and Healthcare BA Training Programs
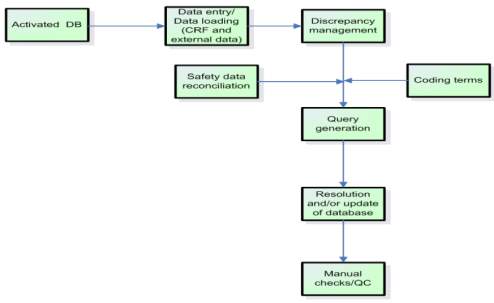
![PDF] Clinical data management: Current status, challenges, and future directions from industry perspectives | Semantic Scholar PDF] Clinical data management: Current status, challenges, and future directions from industry perspectives | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/556d53e90250772fea59270973ccd4118c7550bb/7-Figure1-1.png)
PDF] Clinical data management: Current status, challenges, and future directions from industry perspectives | Semantic Scholar

Clinical Safety Data Management | Pharmacovigilance Consulting Services | Quality Data Services, Inc

Clinical Safety Data Management: Definitions and Standards for Expedited Reporting (ICH E2A) — Clinical Pathways

Book M1: 2022 Mini Pocket-Sized (3" x 5") ICH Guidelines for GCP (E6) – Clinical Research Resources, LLC

Clinical Safety Data Management: Definitions and Standards for Expedited Reporting (ICH E2A) — Clinical Pathways

clinical safety pharmacovigilance certificate program — Clinical Research Blog | Certified Clinical Research Professionals Society - Clinical Research Certification

Clinical Safety Data Management: Definitions and Standards for Expedited Reporting (ICH E2A) Training Certification Course